No, diamonds do not have intermolecular forces in the same way many other substances do. The structure of diamonds is such that each carbon atom is strongly bonded to four other carbon atoms in a three-dimensional network, forming a crystal lattice. These bonds are covalent bonds, which are intramolecular forces within the diamond structure.
Intermolecular forces typically exist between separate molecules, influencing their behavior. Examples include van der Waals forces, dipole-dipole interactions, and hydrogen bonding.
Since it consists of a continuous network of covalent bonds within the crystal lattice, there are no separate molecules, and thus, there are no intermolecular forces between molecules.
Definition of Intermolecular

Intermolecular forces are like interactions between molecules. These forces, unlike the stronger intramolecular forces, don’t need a lot of energy to break. There are three main types of intermolecular forces: van der Waals forces (also called induced dipole forces, London forces, or dispersion forces), permanent dipole-dipole forces, and hydrogen bonding. Before diving into these, let’s quickly revisit bond polarity.
Furthermore, when two atoms share electrons in a covalent bond, the electrons might not be evenly spaced between them. If one atom pulls the electrons more strongly due to differences in electronegativity, a polar bond is formed, and the molecule gets a dipole moment.
The type of intermolecular force a molecule experiences depends on this bond polarity. It’s worth noting that even if a molecule has polar bonds, it might not be polar overall if the dipoles cancel each other out.
Types of Intermolecular Forces
Intermolecular forces play a pivotal role in determining the physical properties of molecules. The strength and nature of these forces vary, primarily depending on the molecule’s polarity. Let’s delve into each type:
- Van der Waals Forces:
- Often termed as London forces, induced dipole forces, or dispersion forces, Van der Waals forces are the most feeble intermolecular forces.
- Electrons within a molecule exhibit constant, random motion, creating temporary dipoles.
- The temporary dipole in one molecule induces a dipole in a neighboring molecule, leading to an attraction known as Van der Waals forces.
- Found universally, irrespective of a molecule’s polarity.
- Strengthen with increasing molecular size due to a larger number of electrons.
Permanent Dipole-Dipole Forces:
- Unlike dispersion forces, permanent dipole-dipole forces are experienced by polar molecules.
- Molecules with non-canceling dipole moments possess a permanent dipole, where one part is partially negatively charged, and another is partially positively charged.
- Oppositely charged dipoles attract, while similarly charged dipoles repel, resulting in stronger forces compared to Van der Waals forces.
- Present between two molecules with permanent dipoles.
Hydrogen Bonding:
- Hydrogen bonding is a powerful intermolecular force, distinct from other dipole-dipole interactions.
- It occurs when a hydrogen atom, covalently bonded to a highly electronegative atom (fluorine, oxygen, or nitrogen), is attracted to another electronegative atom’s lone pair of electrons.
- Only elements with high electronegativity, such as fluorine, oxygen, and nitrogen, can form hydrogen bonds.
- Represented by a dashed line, hydrogen bonds are notably stronger than both permanent dipole-dipole forces and dispersion forces, requiring more energy to overcome.
Examining Intermolecular Forces in Common Molecules
Carbon monoxide (CO) exhibits both permanent dipole-dipole forces and van der Waals forces due to its polar nature. Conversely, carbon dioxide (CO2) experiences only van der Waals forces. Despite having polar bonds, the symmetrical arrangement of its molecules nullifies any permanent dipole moment.
In addition, Methane (CH4) and ammonia (NH3), while similar in size, differ in boiling points. Although both experience comparable van der Waals forces, the substantially higher boiling point of ammonia is attributed to hydrogen bonding—an intermolecular force absent in methane.
Unlike methane, ammonia molecules can form hydrogen bonds, contributing significantly to the increased boiling point. Notably, methane lacks permanent dipole-dipole forces due to its non-polar bonds.
Lastly, understanding these intermolecular forces provides insights into the diverse physical properties of substances, elucidating variations in boiling points and behaviors under different conditions.
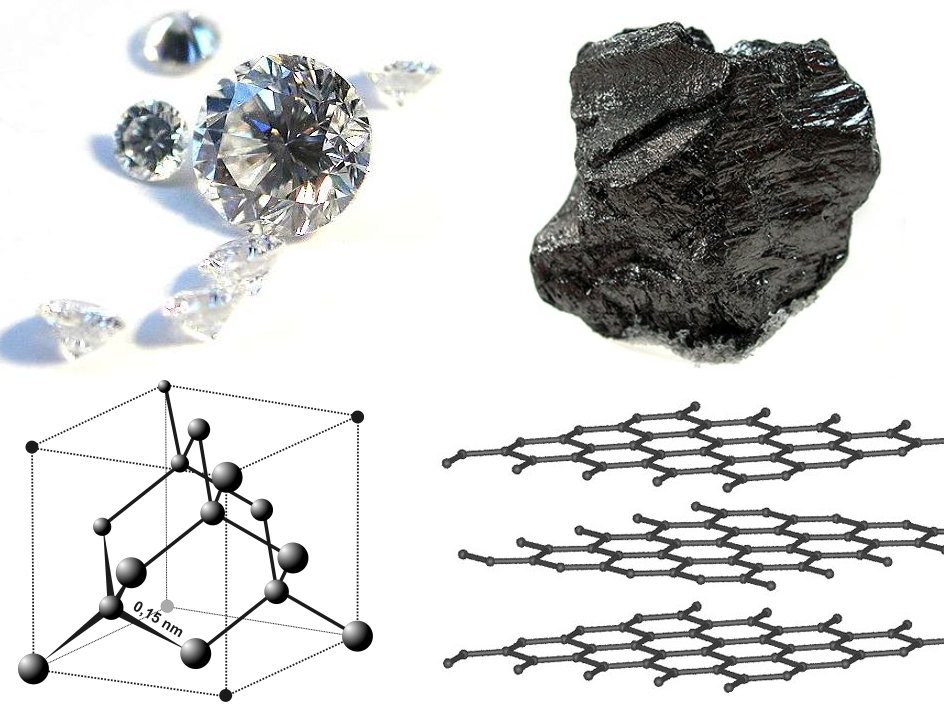
Structure of Diamonds
Diamonds have a unique and well-defined crystal structure, which contributes to their exceptional hardness and other properties. The key features of the diamond structure include
- Carbon atom arrangement:
- Diamonds are composed entirely of carbon atoms.
- Each carbon atom forms four strong covalent bonds with four other carbon atoms.
- The arrangement of carbon atoms is tetrahedral, forming a three-dimensional, repeating pattern throughout the crystal.
- Tetrahedral Structure:
- Each carbon atom in a diamond is bonded to four neighboring carbon atoms, arranged tetrahedrally.
- The tetrahedral arrangement results in a strong and rigid structure.
- Covalent Bonds:
- The bonds between carbon atoms in diamonds are covalent bonds.
- Covalent bonds involve the sharing of electrons between adjacent atoms.
- The shared electrons create a strong network of bonds that extends throughout the entire crystal lattice.
- Crystal Lattice:
- The carbon atoms form a three-dimensional crystal lattice structure.
- The repeating unit of this lattice is a tetrahedron, and these tetrahedra link together to form the overall diamond structure.
- No Free Rotation:
- The tetrahedral arrangement and the strong covalent bonds restrict the movement of atoms.
- Unlike in some other materials, there is no free rotation of atoms or molecules in a diamond crystal.
- Hardness and Stability:
- The strong covalent bonds between carbon atoms make diamonds extremely hard.
- The tetrahedral structure contributes to the stability and rigidity of the crystal.
Diamond Properties: A Closer Look
| Property | Description |
| Hardness | They are super hard, the toughest natural stuff around, thanks to strong bonds between carbon atoms. |
| High Melting Point | Moreover, they don’t melt easily; you need crazy high temperatures (3,500 degrees Celsius or 6,332 degrees Fahrenheit) because breaking their strong bonds takes serious energy. |
| Refractive Index | Diamonds sparkle a lot because they bend and reflect light well, giving them that special brilliance. |
| Dispersion of Light | The colorful sparkle in diamonds, called “fire,” happens because it break down light into different colors. |
| Thermal Conductivity | Diamonds are great at handling heat, which makes them useful in tools for cutting and grinding things in industries. |
| Electrical Insulator | They don’t conduct electricity, unlike some other carbon things like graphite. They’re like electrical insulators. |
| Transparency | They are see-through, letting light shine through, and this clarity makes them look brilliant. |
| Chemical Inertness | doesn’t react much with other stuff; they’re like superheroes against chemical reactions, staying durable and not corroding easily. |
| Density | is pretty heavy for their size, and that’s because the carbon atoms inside them are tightly packed in their crystal structure. |
| Cleavage | don’t break easily in specific directions because of its special crystal structure. They’re good at staying whole. |
FAQ’s
What intermolecular force is present in diamond?
Diamond lacks traditional intermolecular forces due to its unique structure of covalently bonded carbon atoms in a giant network, eliminating the presence of discrete molecules.
Does diamond have strong or weak intermolecular forces?
No, Diamond does not have intermolecular forces as there are no separate molecules. Its strength arises from strong covalent bonds within its crystal lattice.
What substances have intermolecular forces?
Covalent compounds with hydrogen bonds exhibit hydrogen bonds and London dispersion forces, while polar covalent compounds show dipole-dipole attractions and London dispersion forces.
Does water have intermolecular forces?
Yes, water exhibits intermolecular forces, including hydrogen bonds, which are stronger than those in many other substances, influencing the unique properties of water.
Why is diamond hard bonding?
The rigid network of carbon atoms held together by strong covalent bonds makes diamond very hard, making it useful for cutting tools and contributing to its high melting point.
Does diamond have a dipole moment?
Yes, The dipole moment of an ideal bulk diamond is nearly zero, indicating a lack of significant dipole moment in its structure.
What is the weakest intermolecular force?
The weakest intermolecular force is the London Dispersion Force, a temporary attractive force resulting from temporary dipoles in adjacent atoms.
What are the 3 intermolecular forces from weakest to strongest?
In the order of weakest to strongest, the intermolecular forces are dispersion force, dipole-dipole force, and hydrogen bond.
What is the strongest intramolecular force?
Covalent forces are considered the strongest intramolecular forces, responsible for holding atoms together in a lattice structure.
What is the strongest intermolecular force in water?
The strongest intermolecular force in water is hydrogen bonding, accompanied by dipole-induced dipole and London dispersion forces.
Final Words
To sum it up, diamonds are kind of like superheroes among materials. They don’t play by the usual rules of intermolecular forces that other things do. While most substances have these forces between their molecules, diamonds are different. Their crystal structure, where carbon atoms are all tightly connected, means they don’t have separate molecules that experience these forces.
Instead, diamonds get their incredible properties, like extreme hardness and transparency, from the strong bonds within their structure. So, in the world of materials, diamonds are a bit of a rebel, standing out for their unique and exceptional qualities.

